
THE BENEFITS OF TREATING HD CHOREA WITH ONE‑CAPSULE, ONCE‑DAILY INGREZZA
INGREZZA TAKES HD CHOREA CONTROL A STEP FORWARD

In the clinical study, most people began to experience improvement at 2 weeks.* Significant improvement was shown at 12 weeks vs placebo.†
* In a post-clinical study evaluation, 51 of 64 patients (80%) taking INGREZZA 40 mg had at least a 1-point reduction in TMC score at 2 weeks vs before treatment, vs 43 of 60 (72%) for those taking placebo.
† INGREZZA was studied in a 12-week clinical trial. A total of 128 patients participated in the study. Results were based on 64 patients taking INGREZZA 40 mg, 60 mg, or 80 mg. The primary efficacy endpoint was change in TMC from the baseline to maintenance periods.
INGREZZA SIGNIFICANTLY IMPROVED HD CHOREA
People taking INGREZZA experienced greater HD chorea improvement vs placebo as measured by TMC score‡
Most people taking INGREZZA experienced clinically meaningful improvement at 12 weeks§
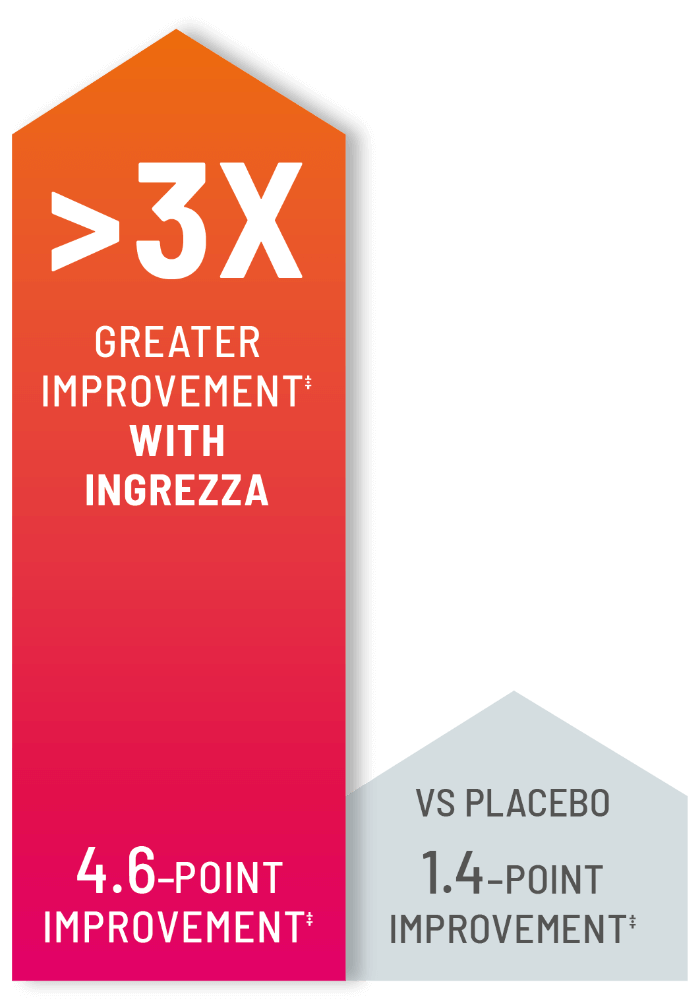
People taking INGREZZA experienced greater HD chorea improvement vs placebo as measured by TMC score‡
Most people taking INGREZZA experienced clinically meaningful improvement at 12 weeks§
‡ 4.6-point improvement seen with INGREZZA (n=58) vs 1.4 with placebo (n=54) in TMC score from the start to the end of the 12-week clinical study.
§ Most patients (57%) experienced clinically meaningful improvements, defined as a 4-point or greater change in TMC score.
Download the INGREZZA Patient Brochure for HD chorea
Learn about Huntington’s Disease (HD) and HD chorea, how INGREZZA works, and the difference it can make in treating uncontrollable movements from HD chorea.

SIGNIFICANTLY MORE PATIENTS AND HEALTHCARE PROVIDERS REPORTED IMPROVEMENT WITH INGREZZA
53% of people
in the clinical study reported their overall HD chorea as “very much improved” or “much improved” with the help of INGREZZA vs 26% for placebo at 12 weeks.‖

‖ 53% of patients (29/55) taking INGREZZA vs 26% of those taking placebo (14/53), based on patient-reported chorea severity scale (PGI-C scale) at 12 weeks vs before treatment.
43% of healthcare professionals
in the clinical study reported HD chorea as “very much improved” or “much improved” with the help of INGREZZA vs 13% for placebo at 12 weeks.¶

¶ 43% of patients (24/56) taking INGREZZA vs 13% of those taking placebo (7/53) based on clinician-reported chorea severity (CGI-C scale) at 12 weeks vs before treatment.
‖ 53% of patients (29/55) taking INGREZZA vs 26% of those taking placebo (14/53), based on patient-reported chorea severity scale (PGI-C scale) at 12 weeks vs before treatment.
¶ 43% of patients (24/56) taking INGREZZA vs 13% of those taking placebo (7/53) based on clinician-reported chorea severity (CGI-C scale) at 12 weeks vs before treatment.
Learn more about the INGREZZA clinical study
128 PEOPLE enrolled
64 PEOPLE
randomly selected
to take INGREZZA
64 PEOPLE
randomly selected
to take placebo
The goal of the study was to see if people taking INGREZZA experienced improvements in their HD chorea as measured by changes in TMC (Total Maximal Chorea) score. Approximately 120 people who completed the study were then enrolled in a 3-year extension to evaluate the long-term safety and effectiveness of INGREZZA in the treatment of HD chorea.


Learn about the potential side effects of INGREZZA
PEOPLE TAKING INGREZZA CAN STAY ON MOST OF THEIR STABLE MENTAL HEALTH MEDICATIONS
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Ready to take HD chorea control a step forward?
The Doctor Discussion Guide has tips for talking to a healthcare provider about your uncontrollable movements and HD chorea treatment options.


Here’s why INGREZZA is unlike other medications approved to treat HD chorea
INGREZZA offers simple dosing that's always one capsule, once daily